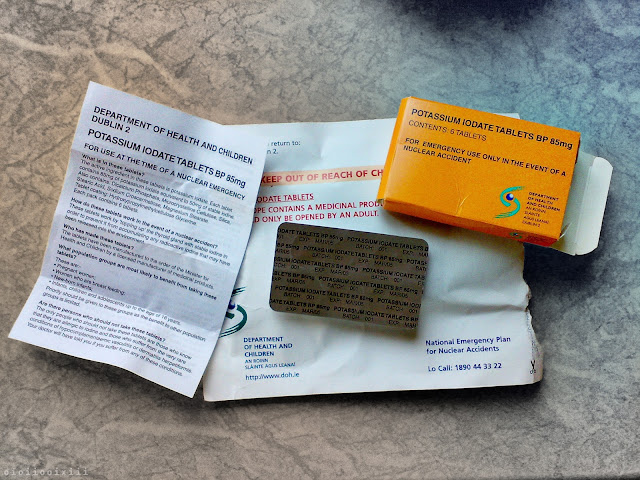
September, 2001: Soon after the "9/11" terrorist attacks in America, a sudden spike in public concern regarding the possibility of an accident, or terrorist attack, at the Sellafield nuclear reprocessing plant causes the Irish government to quickly draw up plans to issue iodine tablets to every household in Ireland.¹
At the time, Ireland was the only European country to take such a measure and it was a source of both national and international ridicule,² even though, some other countries had concerns over the safety of the nuclear facility also.³ Whatever the validity or nativity of such a plan, just like with the 'Millennium Candles' that were set to be delivered to celebrate the year 2000, many households failed to even get delivery of the medicine.
Issued in June 2002,⁴ the original tablets had an expiry date of 2005 and plans to reissue new quantities in 2008 were scrapped.⁵ In 2015, the idea of reissuing iodine tablets was once again quashed when the RPII (Radiological Protection Institute of Ireland) found that the consumption of potassium iodate tablets in Ireland "would not be justified".⁶
From the original 2001 Irish health department press release: "In most nuclear accident situations staying indoors and avoiding consumption of certain foods that may be contaminated would be the advice given."⁷









¹ https://www.rte.ie/archives/2017/0614/882735-iodine-tablets-issued-nationwide/
² https://www.independent.ie/opinion/analysis/bnfl-iodine-tablets-are-of-little-practical-use-26242562.html
³ https://www.theguardian.com/world/2001/aug/09/kursk.russia
⁴ https://www.irishtimes.com/news/households-to-be-posted-iodine-tablets-from-today-1.1060909
⁵ https://web.archive.org/web/20081204005245/http://www.dohc.ie/press/releases/2008/20080403c.html
⁶ https://www.thejournal.ie/iodine-tablets-wont-be-sent-out-again-2157770-Jun2015/
⁷ https://web.archive.org/web/20050218162546/http://www.dohc.ie/press/releases/2001/20010927.html
Other links:
October 4th, 2001:
"Britain to allow Sellafield MOX production"
https://www.rte.ie/news/2001/1003/19313-sellafield/
April 25th, 2006:
Irish TV viewers left concerned after broadcast of "docu-drama" about a nuclear accident at Sellafield:
https://www.independent.ie/irish-news/rte-hit-by-nuclear-disaster-film-fallout-26391049.html
Some content originally published: November 2010